Science : Term 3 Unit 4 : Chemistry in Daily Life
EVALUATION
I. Choose the correct answers
1. A drug effective in the treatment of pneumonia, and bronchitis, is _________
a. Streptomycin
b. Chloramphenicol
c. Penicillin
d. Sulphaguanidine
[Answer: (c) Penicillin]
2. Aspirin is ___________
a. Antibiotic
b. Antipyretic
c. Sedative
d. Psychedelic
[Answer: (b) Antipyretic]
3. __________ are that neutralize stomach acid.
a. Antacid
b. Antipyretic
c. Analgesic
d. Antihistanics
[Answer: (a) Antacid]
4. The lowest temperature at which a substance catch the fire is called its ________
a. Boiling point
b. Melting point
c. Critical temperature
d. Ignition temperature.
[Answer: (d) Ignition temperature]
5. Which is the hottest part in the flame of candle _________
a. Blue
b. Yellow
c. Black
d. Way part
[Answer: (a) Blue]
II. Fill in the blanks.
1. Penicillin was first discovered by Alexander Fleming
2. World ORS Day is July 29
3. Combustion is a chemical reaction in which and substance react with oxidizing agent
4. In the presence of water, the ignition temperature of paper is not reached
5. Fire produced by oil cannot be controlled by water
III True or False – If False give the correct answer
1. Antibiotics does work for viruses like cold and Answer: False.
Correct statement: Antibiotics does not work for viruses like cold and flu.
2. Analgesics are the substances that lower the temperature during fever. Answer: False.
Correct statement : Antipyretic are the substances that lower the temperature during fever.
3. All fuels form flame. Answer: False.
Correct statement: All fuels do not form flame.
4. Oxygen is necessary for combustion Answer: True.
5. Burning wood and coal causes pollution of air. Answer: True.
IV Match the following
1. Antipyretic – reduce pain
2. Analgesic – reduce body temperature
3. Antacid – spontaneous combustion
4. Phosphorus – ORS Solution
5. Carbon – d oxide i – – leads to respiratory problem.
Answer:
1. Antipyretic – Reduce body temperature
2. Analgesic – Reduce pain
3. Antacid – ORS Solution
4. Phosphorus – Spontaneous combustion
5. Carbon – di – oxide – Leads to respiratory problem.
V Analogy
1. Inner zone of flame : : Black, outer zone of flame : : Blue
2. Tincture : : Antiseptic , cistamine : : Chemical messenger.
VI Very short answer
1. First viral disease detected in human being was : Yellow fever. (Yellow fever / dengue fever)
Answer: Yellow fever.
2 :—————, :————— , :————–are called green house gases (Fleming / lenis pastor)
Answer: CO2, Methane, Chlorofluorocarbons.
3. Name a substance which can be used as an antiseptic as well as disinfectant?
Answer: Garlic, Turmeric, Aloe vera.
4. What are the main constituents of dettol?
Answer: Mixture of chloroxylenol and terpineol
5. Name the unit in which the calorific value of a fuel is expressed?
Answer: KJ/Kg.
6. How many types of combustion are there?
Answer:
(i) Rapid combustion
(ii) Spontaneous combustion
(iii) Explosion
7. What are the essential requirements for producing fire?
Answer: Fuel, Heat and Oxygen.
VII Short Answer Questions
1. Why should not medicines be taken without consulting doctors?
Answer: One should not take medicines without consulting doctors because if a wrong medicine is accidently eaten for a disease, it may not cure the disease but actually can have harmful side effects to the body.
2. Why do antiseptics differ from disinfectants? Give one example of each.
Answer: Difference between Antiseptic and Disinfectants

Antiseptic
1. All antiseptic are disinfectants.
2. It can be applied on the live tissue
3. E.g. skin / Mucous
Disinfectants
1.All disinfectants are not antiseptic
2. It can be apply on in animate object
3. E.g. Surface, lab working tables, floor.
3. What is ignition temperature?
Answer: The minimum temperature at which a substance catches fire and burns is called its ignition temperature.
4. If 4.5kg of fuel is completely burnt and amount of heat produced stands measured at 1, 80,000 KJ what is the calorific value.
Answer: Amount of fuel = 4.5 kg
Heat produced = 1,80,000 kJ
Calorific value = ?
Solution :
Calorific Value = Heat produced /Amount of fuel = 1,80,000 / 4 .5 = 40,000
Calorific Value = 40,000 KJ / kg.
VIII Answer in Detail
1. Explain briefly about antibiotic and analgesic?
Answer:
Antibiotics:
(i) Many micro organisms and plants synthesize chemicals which are toxic in nature to protect them from invading organisms.
(ii) Those biosynthesized chemicals can be isolated from the plants/micro organisms and was used as medicines against infectious diseases, these substances were called as antibiotics.
(iii) Ex: Chloramphenicols, tetracyclines, Penicillin derivatives, cephalosporin’s and their derivatives.
(iv) The world’s first antibiotic penicillin was discovered by Dr. Alexander Fleming.
Analgesics:
(i) Analgesics or pain killers that react like the pain-suppressing chemicals released by the body.
(ii) They suppress the feeling of ‘pain’.
(iii) This analgesics drug selectively relieves pain by acting either in CNS (Central Nerves System) or on peripheral pain mechanism, without significantly altering consciousness.
2. Make labeled diagram of a candle flame.

IX Picture based question
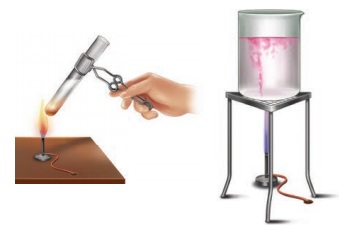
Arul and Aakash were doing an experiment in which water was to be heated in a beaker. Arul kept the beaker near the wick in the yellow part of candle flame. Aakash kept the beaker in the outer most part to the flame. Whose water will get heated in a shorter time?
Answer: The water heated by Akash will get heated in a shorter time because he kept his beaker near the hottest (non luminous) zone of the flame. But Arul kept the beaker in the luminous zone which is moderately hot. So, it will take longer time.
Student Activities
ACTIVITY
What happens when you add with these chemicals?
Sugar + Potassium permanganate+ Glycerin.

Answer:
(i) After adding sugar, potassium permanganate and glycerin to the dish, immediately step back because spark and solid potassium permanganate will be expelled from the dish.
(ii) When potassium permanganate mixes with glycerin, a redox reaction starts. This reaction starts out really slow, but produces a lot of heat, so it will start to speed up bit by bit. As the potassium permanganate oxidises the sugar, it will speed up more and more until it finally starts to smoke and after that it will ignite.
Anesthetics
The first local anesthetic was cocaine was isolated from coca leaves by Albert Niemannin
1. Dettol
Mixture of chloroxylenol and terpincol

2. Tincture
Iodine + 2 to 3% alcohol – Water mixture Soap, Iodoform,
phenolic solutions, ethanol, Boric acid, are examples.
Fact
Fire chemical Reaction
Oxygen + Heat + Fuel = Fire
