Science : Chapter 10 : Changes Around Us
TEXTBOOK EXERCISE
I. Choose the best answer.
1. Burning of paper is a __________ change.
a) physical
b) chemical
c) physical and chemical
d) neutral
[Answer: (b) chemical]
2. Burning of matchstick is an example for chemical reaction caused by __________
a) contact in physical states
b) electricity
c) light
d) catalyst
[Answer: (a) Contact in physical states]
3. __________ undergoes rusting.
a) Tin
b) Sodium
c) Copper
d) Iron
[Answer: (d) iron]
4. The pigment responsible for browning of apples is __________.
a) hydrated iron (II) oxide
b) melanin
c) starch
d) ozone
[Answer: (b) melanin]
5. Brine is a concentrated solution of__________.
a) sodium sulphate
b) sodium chloride
c) calcium chloride
d) sodium bromide
[Answer: (b) sodium chloride]
6. Limestone contains __________ mainly.
a) calcium chloride
b) calcium carbonate
c) calcium nitrate
d) calcium sulphate
[Answer: (b) calcium carbonate]
7. Which of the following factor induces electrtolysis?
a) Heat
b) Light
c) Electricity
d) Catalysis
[Answer: (c) electricity]
8. In Haber’s process of producing ammonia__________ is used as a catalyst.
a) nitrogen
b) hydrogen
c) iron
d) nickel
[Answer: (c) iron]
9. Dissolved gases like sulphur dioxide and nitrogen oxides in rain water causes__________
a) acid rain
b) base rain
c) heavy rain
d) neutral rain
[Answer: (a) Acid rain]
10. __________ is/are responsible for global warming.
a) Carbon dioxide
b) Methane
c) Chlorofluorocarbons
e) Carbon dioxide, Methane, Chlorofluorocarbons
[Answer: (d) Carbon di oxide, methane, chlorofluoro carbons]
II. Fill in the blanks.
1. Photosynthesis is a chemical reaction that takes place in the presence of sunlight.
2. Iron objects undergo rusting when exposed to water and oxygen.
3. Ammonia is the basic material to manufacture urea.
4. Electrolysis of brine solution gives chlorine and hydrogen gases.
5. Catalyst is a chemical substance which alters the speed of a chemical reaction.
6. Poly phenol oxidase or tyrosinase is the enzyme responsible for browning of vegetables and fruits.
III. Say true or false. If false, correct the statement.
1. A chemical reaction is a temporary reaction. Answer: False.
Correct statement: A chemical reaction is permanent.
2. Decomposition of lead nitrate is an example for a chemical reaction caused by light. Answer: False.
Correct statement: Decomposition of lead nitrate is an example for a chemical reaction based on heat.
3. Formation of slaked lime from quicklime is an endothermic reaction. Answer: False.
Correct statement: Formation of slaked limed from quicklime is a exothermic reaction.
4. CFC is a pollutant. Answer: True.
5. Light energy may come out due to chemical reactions. Answer: True
IV. Match the following.
a.
Rusting – Photosynthesis
Electrolysis – Haber’s process
Thermolysis – Iron
Food – Brine
Catalysis – Decomposition oflimestone
[Answer: (1 – c, 2 – d, 3 – e, 4 – a, 5 – b]
1. Rusting – (c) Iron
2. Electrolysis – (d) Brine
3. Thermolysis – (e) Decomposition of limestone
4. Food – (a) photosynthesis
5. Catalysis – (b) Haber’s process
b.
Spoilage – Decomposition
Ozone – Biocatalyst
Tarnishing – Oxygen
Yeast – Chemical reaction
Calcium oxide – Food
[Answer: (1 – e, 2 – c, 3 – d, 4 – b, 5 – a)]
Spoilage – (e) Food
Ozone – (c) oxygen
Tarnishing – (d) chemical reaction
Yeast – (b) biocatalyst
Calcium Oxide – (a) Decomposition
V. Answer briefly.
1. Define a chemical reaction.
Answer: Chemical changes are otherwise called as chemical reactions, because one or more substances (Reactants) undergo a reaction to form one or more new substances (Products).
Reactant(s) ____→ Product(s)
2. Mention the various conditions required for a chemical reaction to occur.
Answer: The conditions required for a chemical reaction to take place :
(i) Physical contact
(ii) Solution of reactants
(iii) Electicity
(v) Light
(iv) Heat
(vi) Catalyst
3. Define catalysis.
Answer: Chemical substance used to alters the speed of the reaction is called catalyst and the process is called catalysis.
4. What happens when an iron nail is placed in copper sulphate solution?
Answer: When iron nail is dipped in the solution of copper sulphate, copper is displaced by iron and forms ferrous sulphate. Thus, the blue colour of copper sulphate changes into green because of formation of ferrous sulphate.
5. What is pollution?
Answer: Unwanted change in physical, chemical and biological properties of the environment. This is termed as pollution.
6. What is tarnishing? Give an example.
Answer: Tarnishing of metal articles : Shiny metal surfaces and other articles lose their shining appearance due to chemical reactions on the surface. For example, silver articles become black on exposure to atmospheric air. Similarly, brass vessels which contain copper as one of constituents develop a greenish layer on exposure to air for a long time. This is due to a chemical reaction between copper and moist air to form basic copper carbonate and copper hydroxide.
7. What happens to the brine during electrolysis?
Answer: A concentrated solution of sodium chloride called BRINE is electrolysed to produce chlorine and hydrogen gases along with sodium hydroxide. This is a very important reaction to produce chlorine industrially.
8. On heating, calcium carbonate gives calcium oxide and oxygen. Is it an exothermic reaction or an endothermic reaction?
Answer: Endothermic reaction.
9. What is the role of a catalyst in a chemical reaction?
Answer: Substances used to alter the speed of a chemical reaction, are called catalysts.
For example, metallic iron is used as a catalyst in the manufacture of ammonia by Haber’s process.
10. Why photosynthesis is a chemical reaction?
Answer:
(i) Photosynthesis is a process in which light energy from the sun is used by the plants to prepare starch from carbon dioxide and water.
(ii) The sunlight uses the chemical reaction between carbon dioxide and water, which finally ends up in the production of starch (photo means light and synthesis means production). These chemical reactions in used by light are called as photochemical reactions.
VI. Answer in detail.
1. Explain the environmental effects of chemical reactions?
(a) Pollution :
(i) Environment is the place around you that comprises both living and non living things.
(ii) Due to human activities like industries, increasing number of automobiles etc our environment is badly affected now-a-days.
(iii) So, there is an unwanted change in physical, chemical and biological properties of the environment. This is termed as pollution.
(iv) The substances which cause these changes are called pollutants.
(v) Due to increasing human activities lot of chemical substances are produced artificially which harm all the living and non living things.
(b) Rusting:
(i) Steel benches and tables turn into reddish brown during rainy season.
(ii) This is because when the iron metal comes into contact with water and oxygen, it undergoes a chemical reaction called RUSTING.
(c) Tarnishing of metal articles :
(i) Shiny metal surfaces and other articles lose their shining appearance due to chemical reactions on the surface.
(ii) For example, silver articles become black on exposure to atmospheric air.
(iii) Similarly, brass vessels which contain copper as one of constituents develop a greenish layer on exposure to air for a long time.
(iv) This is due to a chemical reaction between copper and moist air to form basic copper carbonate and copper hydroxide.
2. Explain how food items are spoiled by chemical reactions?
Answer:
(i) Spoilage of food and vegetables : Food spoilage may be defined as any change that causes food unfit for human consumption. The chemical reactions catalyzed by the enzymes result in the degradation of food quality such as development of bad taste and odour, deterioration and loss of nutrients.
E.g. 1: Rotten eggs develop a bad smell due to formation of hydrogen sulphide gas.
E.g. 2 : Decaying of vegetables and fruits due to microbes.
(ii) Rancidity of fishes and meat:
Fishes and meat containing high levels of polyunsaturated fatty acids that undergo oxidation causes bad odour when exposed to air or light. This process is called rancidity.
(iii) Apples and fruits turn brown when cut:
(i) Apples and some fruits turn brown due to chemical reaction with oxygen in air. This chemical reaction is called browning.
(ii) The cells of apples, fruits and other vegetables contain an enzyme called polyphenol oxidase or tyrosinase, when come in contact with oxygen catalyses a biochemical reaction of plants, phenolic compounds to brown pigments known as melanins.
3. Explain any three conditions that is required for a chemical reaction to take place. Give example.
Answer: Conditions required for a chemical reaction to take place :
Chemical reactions can be done through ;
(i) Physical contact
(ii) Solution of reactants
(iii) Electricity
(iv) Heat
(v) Light
(vi) catalyst
(i) Chemical Reactions Based on Solution of Reactants :
When we mix two substances (Reactants) in solution form, the chemical reaction takes place to form new substances (Products).
Example :
(1) Take small amount of solid silver nitrate and sodium chloride in a test tube. The reactants in solid state have no reactions.
(2) Dissolve the same reactants in water in separate test tubes.
(3) Mix both the solutions. Silver nitrate solution reacts with sodium chloride solution to form a white precipitate of silver chloride and sodium nitrate solution.
(4) From the above reaction, we infer that some chemical reactions proceed only in solution form not in solid form.
(ii) Chemical Reaction Based on Electricity :
(1) When electricity is passed through water containing small amounts of sulphuric acid, hydrogen and oxygen gases are liberated.
(2) Similarly, a concentrated solution of sodium chloride called BRINE is electrolysed to produce chlorine and hydrogen gases along with sodium hydroxide.
(3) This is a very important reaction to produce chlorine industrially.
(4) From the above two reactions, we infer that some chemical reactions proceed only by the passage of electricity.
(5) Hence, such reactions are called as electrochemical reaction or electrolysis.
(iii) Chemical Reactions Based on Light:
Sunlight is important not only for us but also for plants as welll. As you know photosynthesis is a process in which light energy from the sun is used by the plants to prepare starch from carbon dioxide and water.
Example : The sunlight uses the chemical reaction between carbon dioxide and water, which finally ends up in the production of starch (photo means light and synthesis means production). These chemical reactions is used by light are called as photochemical reactions.
VII. Higher order thinking questions.
1. Explain the role of yeast in making cakes and buns in a bakery?
Answer:
(i) Yeast is a key ingredient in the production of baked goods.
(ii) Yeast is a bio-organic catalyst
(iii) Carbon dioxide is generated by the yeast as a result of the breakdown of fermentable sugars in the dough and makes cakes to rise, when baked, giving it the required and soft texture.
2. Burning of fossil fuels is responsible for global warming. Justify the statement.
Answer: The combustion of fossil fuels also releases a large amount of carbon dioxide into the atmosphere. Carbon dioxide is a greenhouse gas which is responsible for global warming. Humans bum fossil fuels, releasing huge amounts of carbon pollution and trapping more and more heat in the atmosphere.
3. Discuss how acid rain occurs due to emission of smoke from vehicles and industries?
Answer: (i) Rain becomes acidic in nature due to the presence of certain pollutants in the air released by cars and industrial processes.
(ii) Acid rain is caused by emissions of sulphur dioxide and nitrogen oxide which react with the water molecules in the atmosphere.
4. Is rusting good for iron materials? Explain.
Answer: No. Rusting is not good for iron materials. Rust is a form of iron oxide. It occurs when iron combines with the oxygen in the air causing it to corrode. Rust can affect iron and its alloys. It makes them weaker, by replacing the strong iron with flaky powder.
5. Do all the fruits and vegetables undergo browning? Explain.
Answer: No, not all the fruits. Enzymic browning can be observed in fruits such as apricots, pears, bananas, grapes and avocados and vegetables such as aubergines, potatoes, lettuce. The enzyme polyphenol oxidase or tyrosinase that when in contact with oxygen catalyses a biochemical reaction of plants phenolic compounds to brown pigments known as melanin, which results in the fruit or vegetable turning brown.
6.Classify the following day to day activities based on chemical reactions by physical contact, solutions of reactants, heat, light, electricity and catalyst.
a ) Burning of crackers during festivals
b) Fading of coloured clothes on drying under sunlight.
c) Cooking of eggs.
d) Charging of batteries.
Answer:
(a) Chemical reactions by physical contact
(b) Chemical reactions based on heat
(c) Chemical reactions based on heat
(d) Chemical reactions based on electricity
VIII. Value Based Questions.
1. Kumar is going to build a house. To purchase the iron rods required for construction, he visited an iron and steel shop nearby. The seller showed him some iron rods which are fresh and good. He also showed him little older iron rods which are brownish in appearance. The price of fresh rods is more than the older ones. The seller also gave some offer to older ones. Kumar’s friend Ramesh advised him not to buy the cheaper rods.
a) Is Ramesh right in his suggestion?
b) Could you explain the reason for his suggestion?
c) What are the values shown by Ramesh?
Answer:
(i) Yes, Ramesh is right in his suggestion because old iron rods were rusted.
(ii) The older rods are brownish and they are rusted. Rusting destroys the quality of the iron rods and harmful for construction.
(iii) Responsible behaviour, correct decision, caring and awareness.
2. Palanikumar is a Lawyer. He lives in a luxurious flat. Due to high rent, he wants to shift his residence to a place where he has a chemical industry nearby. There the rent is very cheap and the area is less populated also. His son Rajasekar, studying VIII, does not like this and likes to go to some other place.
a) Is Rajasekar right in his attitude?
b) Why did he refuse to go there?
c) What are the values shown by Rajasekar?
Answer:
(i) Yes, Rajasekar is more concerned over his family health than saving money.
(ii) Rajasekar know that the air pollution from nearby-chemical industry would be harmful and affect his family health.
(iii) Environmentally sensitive, awareness, caring and correct decision.
Student Activities
Activity 1
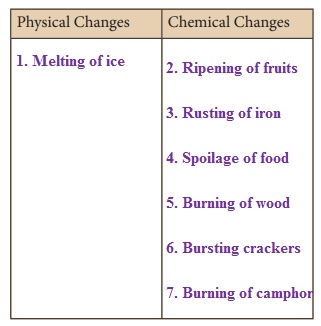
Adithya wants to classify the following changes as physical or chemical. Can you help him?
1. Melting of ice
2. Ripening of fruits
3. Rusting of iron
4. Spoilage of food
5. Burning of wood
6. Bursting crackers
7. Burning of camphor
Activity 2
Take two test tubes and couple of rust free iron nails. In one test tube pour some water and put an iron nail. Keep the test tube opened for few days. Take another test tube and pour some water along with some coconut oil. Now, place the second iron nail. Leave the set up for a few days. Observe the changes and record them. Which iron nail gets rusted and why?
Activity 2
Take two test tubes and couple of rust free iron nails. In one test tube pour some water and put an iron nail. Keep the test tube opened for few days. Take another test tube and pour some water along with some coconut oil. Now, place the second iron nail. Leave the set up for a few days. Observe the changes and record them. Which iron nail gets rusted and why?
Activity 3
Buy some fresh yeast from a grocery shop. Prepare a paste of wheat flour with water in a vessel. Add some yeast and leave the vessel closed for few hours under sunlight. Observe the changes closely. What do you infer?
Activity 4
Take two clean test tubes. Take sulphuric acid in one test tube and a solution of sodium hydroxide in another tube. Slowly and carefully add sodium hydroxide solution to sulphuric acid. Touch the sides of test tube. What do you feel? What do you infer?
Activity 5
Take a clean test tube. Add some dilute hydrochloric acid. Drop a piece of magnesium or a piece of zinc metal. What do you see? Now bring a burning match stick near the mouth of the test tube. What do you hear? What do you infer?
More to know
The head of a matchstick contains potassium chlorate and antimony trisulphide. The sides of the matchbox contain red phosphorous.
The term electrolysis was introduced by Michael Faraday 19th in the century. The word electrolysis is acombinationoftwo terms‘electron’ and ‘lysis’. Electron is relatedto electricity and lysis means decomposition

More to Know
Chemical reactions accompanying evolution of heat are called exothermic reactions. Whereas chemical reactions which involve absorbtion of heat are called endothermic reactions.
Limestone is the raw material for quicklime, slaked lime and cement.

The ultraviolet rays from the sun break ozone (O3) molecules in the stratosphere into molecular oxygen and atomic oxygen. This atomicoxygen again combines with molecular oxygen to form Ozone.
More to Know
Photochemistry is the branch of chemistry which deals with chemical reactions involving light.
Enzymes and yeasts are called biocatalysts.















