Science : Term 1 Unit 2 : States of Matter
Unit 2
States of Matter

Learning Objectives
After learning this lesson, students will be able to
* know the matter surrounding us
* differentiate between solids, liquids and gases
* conduct simple experiments
* observe the properties of matter
* describe the nature of the material
Matter
Teacher : Leela, look at the picture and list out the things you see in it.
Leela : Yes madam. Sun, river, boat, house, tree, car, birds, …
Teacher : Very good. There are many things in this picture. Some of them are natural and some are man-made.

You can see a number of things around you. Everything you can see and touch is made up of matter. Anything that occupies space and has mass is called matter
Let us Do
List out the some of the matter around you.
Answer: 1. Book 2.Table 3.Cloud 4. Tree
More to know
What is mass?
Mass is a measure of how much matter is in an object.
* The air we breathe, the food we take, and the water we drink all have matter in them.
Do you know that even you are made up of matter?
The space occupied by an object is called its volume.
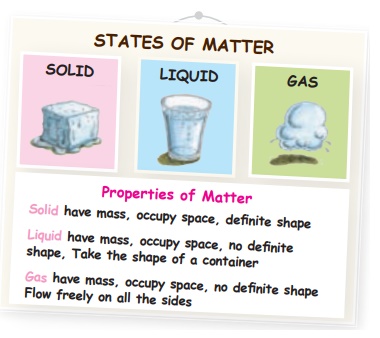
1. States and properties of Matter
Matter can exist as solid, liquid or gas.

i. Solid
* rigid
* fixed shape
* fixed volume
ii. Liquid
* not rigid
* no fixed shape
* fixed volume
iii. Gas
* not rigid
* no fixed shape
* no fixed volume
I. SOLIDS
Let us Try
Press a wooden pencil. Is the pencil hard? Yes / No.
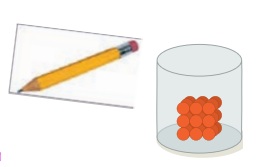
Solids are things that have a definite shape and volume. They occupy a fixed space. The particles in solids are packed very tightly. So they cannot move freely. Their shape can be changed only when we break or cut them.
Some examples for solids are given below.

II. LIQUIDS
Let us Do
1. Place 4 1L bottles of different shapes on the table.
2. Take a bucket with water.
3. Call one child to hold the empty bottles and the other to fill water into them using a paper cup.
4. Ask the other children to fill the table as given below.
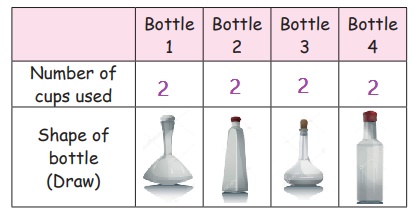
Did each bottle need the same number of cups to get filled?
We can see that water takes up the same space in each bottle and the shape of the water is same as the shape of the bottle.
Think Zone
1. What is the shape of water in your bottle?
The water takes up the shape of my bottle.
2. What happens if you pour water on the floor or table?
The water flours.
Look at these pictures
Here we can see that the shape of the liquid is determined by the shape of the container.
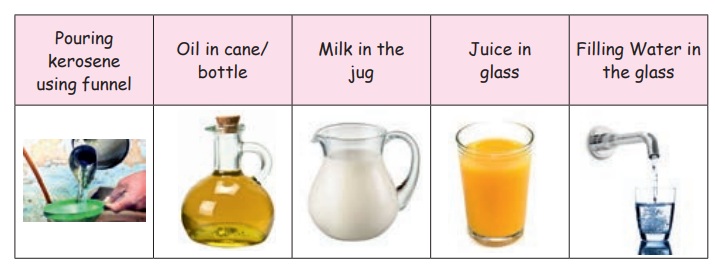
Liquids are the things that do not have a definite shape but occupies space. They have a definite volume. They take the shape of the container in which they are filled. The water moves from one place to another. This is because the matter in liquid are loosely packed. So, liquids can flow freely.

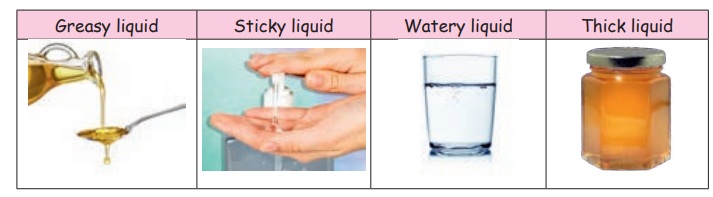
Let us Touch and Feel
Different types of liquids are placed in separate containers. Students are allowed to touch and feel every type of liquid. They are asked to tell the type of the liquids on the basis of their stickiness/concentration.
III. GASES
When a perfume is sprayed or an agarbatti is lighted, the fragrance spreads all around the room. How?
The matter in gases are very loosely packed. So they can move around freely in all directions. Hence, gases do not have a definite shape and do not occupy a definite space or volume.
Most of the gases are colourless. But when they are mixed with solid particles they show distinct colours.

Think Zone
Cooking gas in gas-cylinder has a smell. Why?
Because Ethyl mercaptan is added with LPG to find the leakage.
Here are some examples for gases

Let us Do
Say whether it is Solid or Liquid or Gas (Put ‘S’ for Solid, ‘L’ for Liquid and ‘G’ for Gas).
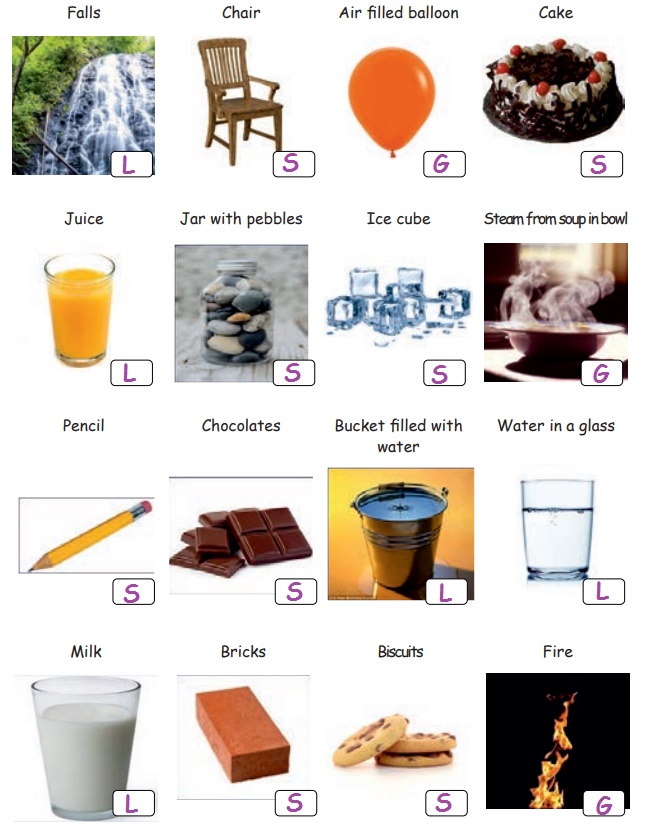
Let us Read and Complete the table
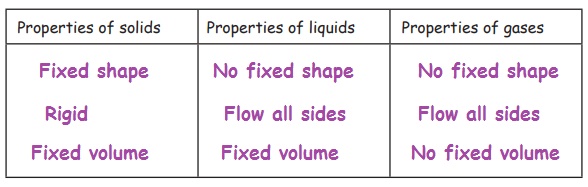
Here are some properties of matter:
Fixed shape, No fixed shape, Fixed volume, No fixed volume, Flow all sides, Rigid

Copy the following table. Write each property in the correct column of the table. Some properties may belong to more than one column.
2. Change in States of Matter
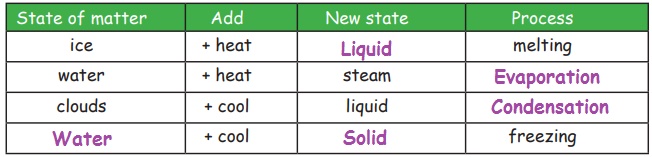
Matters change their state as the temperature changes. Solid changes into liquid and liquid changes into gas on heating. Gas becomes liquid and liquid becomes solid on cooling.
Melting
Change of solid into liquid on heating is called melting. For example, if ice (solid) is heated, it willchange into water (liquid).

Let us Do
* Take some ice cubes in a container. Heat the container and observe the changes.
Ice cubes melt.
* Take some cheese in a container. Heat the container and observe the changes.
Cheese melts.
* Take some jaggery in a pan. Heat the pan and observe the changes.
Jaggery melts.
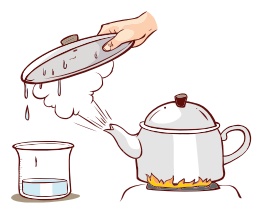
Evaporation
Change of liquid into vapour on heating is called Evaporation. For example, if water is heated, it willchange into steam.

Freezing
Change of liquid into solid on cooling is known as freezing. For example, water (liquid) poured in ice-tray and placed in the freezer (fridge), gets cooled and becomes ice (solid).

Condensation
Changes of gas into liquid on cooling is called condensation. For example; clouds (gas) on coolingcondense and fall as rain (liquid)
Let us think
What makes the coconut oil freeze in winter season?

In winter season room temperature will drop down almost below 20°C which is less than the freezing point of coconut oil. So it freeze in winter season.
Complete the table
Think and answer
One of these cans was in the fridge and the other was not.
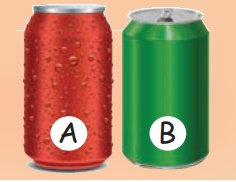
a) Which can was taken from the fridge?
Can A.
b) How do you know?
Water droplets appear on the can A.
c) How did water droplets appear on the can A?
Because of condensation.
d) Why are there no water droplets on can B?
Can B is not kept in the fridge.
Let us Observe
Take a balance. Keep an air filled football in one plate and an empty football in another plate . What happens?
Air filled ball goes down. It is because air has mass.

Do you know
Air is a mixture of gases. You can feel the presence of air when the wind blows.
Let us Prepare – Anchor chart
1. Cut a chart into three pieces each of 15cm × 10 cm.
2. Write the properties of solid, liquid and gas in separate sheets.
3. Draw pictures related to the points.
4. Design the sheets with colourful borders.
5. Paste all the sheets in a large chart paper. Your anchor chart is ready. Hang it on the wall.
Let us Understand
* Keep a stone on the floor. Does it move by itself?
No
* Pour a mug of water on the floor. Does the water flow? Does it flow in one direction?
Yes, the water flows. It flows in all direction.
* Take an air filled balloon. Prick it with a needle. Does the air rush out?
Yes
* Fill an open vessel with water. Press the surface of the water with your hands. How do you feel?
Water overflows.
4. Materials Used / Not Used For Heating
Look at the pictures. Write down what you see.
(Wood, Leaves, Paper)

Fuels
* Paper, firewood, dried leaves and charcoal can be burnt.
* Liquids like kerosene, petrol and diesel also burn on heating.
* Domestic gas burns and helps in cooking.
Substances when burnt give out heat. But in some substances, the heat released is very low. Thus, these are not used for heating purpose.
Substances that give out more heat while burning are used for heating purpose. These substances are called fuels.

Match the following.

Do you know
Electrical energy is also used as fuel for cooking and transporting.
EVALUATION
I. Indicate whether the following statements are true or false.
1. Solids have a definite volume. (True)
2. Liquids can not flow. (False)
3. We can melt any substance by cooling it. (False)
4. Liquids can take the shape of the container. (True)
5. Gases have a definite shape or volume. (False)
6. Matter changes its state when heat is added or removed. (True)
7. A fuel is a substance which gives heat energy on burning. (True)
II. Fill in the blanks. (Evaporation, Mass, Water, Solid, Stone, Freezing)
1. The measure of matter in an object is called Mass.
2. Change of liquid into vapour on heating is called Evaporation.
3. An example for liquid is Water.
4. The change of liquid into solid on cooling is known as Freezing.
5. An example for solid is Stone.
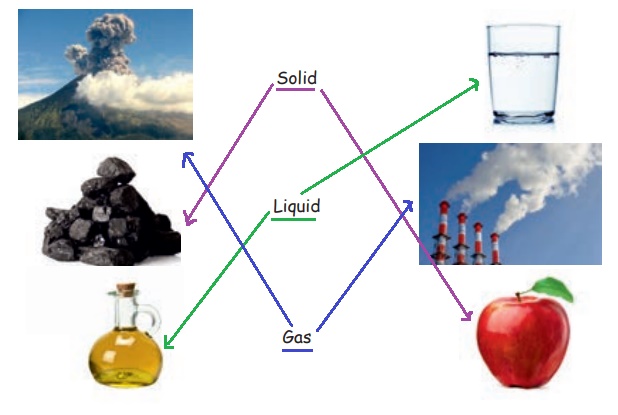
III. Draw a line to match the objects and their state of matter.
IV. Answer in a word or two.
1. Which of these is a solid: wood or juice?
Answer : Wood.
2. Which of these is hard : a sponge or a glass or a cloth?
Answer : a glass.
3. What are three states of matter?
Answer : Solid , Liquid, Gas.
4. Name three substances which can change to liquid when they are heated?
Answer : Ice, Cheese, Jaggery.
5. In which state of matter the particles are very close to each other?
Answer : Solid State.
6. What state of matter is rain?
Answer : Liquid state
7. Which among the state of matter has definite size but no definite shape?
Answer : Liquid state
8. What would cause a liquid to turn into a solid?
a) Pouring it into a container
b) Heating it until it boils
c) Cooling it until it freezes
d) Keeping its temperature the same
Answer : c) Cooling it until it freezes
9. What are some properties of pencil?
Answer :
* Pencil is rigid
* It has definite shape and volume.
* It occupies a fixed space.
V. Find me. ( Liquid, Water, Wood)
1. I am a five letter word. I am an essential need for your life. I remain in all the three states of matter. Who am I ?
Answer :I am water.
2. I am a solid. I am obtained from the trees. I am useful for heating. Who am I?
Answer :I am wood.
3. I am one among the three states. I have loosely arranged particles. I become vapour on heating. Who am I ?
Answer :I am liquid.
VI. Describe the word in one sentence.
1. Solid:
* Solids are things that have definite shape and volume.
2. Liquid:
* Liquids are things that do not have a definite shape but occupies space.
3. Melting:
* Change of solid into liquid on heating is called melting.
4. Evaporation:
* Change of liquid into vapour on heating is called evaporation.
5. Freezing:
* Change of liquid into solid on cooling is known as freezing.
VII. Which change of state is taking place in each description below? Use these words.
Freezing, Evaporation, Condensation, Melting
a) An ice cube turning to water Melting
b) Water turning to ice in a freezer Freezing
c) Change of liquid into vapour on heating Evaporation
d) A bathroom mirror misting up Condensation
Let us Do
List out the some of the matter around you.
Answer: 1. Book 2.Table 3.Cloud 4. Tree
Let us Do
1. Place 4 1L bottles of different shapes on the table.
2. Take a bucket with water.
3. Call one child to hold the empty bottles and the other to fill water into them using a paper cup.
4. Ask the other children to fill the table as given below.

Did each bottle need the same number of cups to get filled?
We can see that water takes up the same space in each bottle and the shape of the water is same as the shape of the bottle.
Think Zone
1. What is the shape of water in your bottle?
The water takes up the shape of my bottle.
2. What happens if you pour water on the floor or table?
The water flours.
Think Zone
Cooking gas in gas-cylinder has a smell. Why?
Because Ethyl mercaptan is added with LPG to find the leakage.
Let us Do
Say whether it is Solid or Liquid or Gas (Put ‘S’ for Solid, ‘L’ for Liquid and ‘G’ for Gas).

Let us Read and Complete the table
Here are some properties of matter:
Fixed shape, No fixed shape, Fixed volume, No fixed volume, Flow all sides, Rigid

Copy the following table. Write each property in the correct column of the table. Some properties may belong to more than one column.

Let us Do
* Take some ice cubes in a container. Heat the container and observe the changes.
Ice cubes melt.
* Take some cheese in a container. Heat the container and observe the changes.
Cheese melts.
* Take some jaggery in a pan. Heat the pan and observe the changes.
Jaggery melts.
Let us think
What makes the coconut oil freeze in winter season?

In winter season room temperature will drop down almost below 20°C which is less than the freezing point of coconut oil. So it freeze in winter season.
Complete the table

Think and answer
One of these cans was in the fridge and the other was not.

a) Which can was taken from the fridge?
Can A.
b) How do you know?
Water droplets appear on the can A.
c) How did water droplets appear on the can A?
Because of condensation.
d) Why are there no water droplets on can B?
Can B is not kept in the fridge.
Let us Understand
* Keep a stone on the floor. Does it move by itself?
No
* Pour a mug of water on the floor. Does the water flow? Does it flow in one direction?
Yes, the water flows. It flows in all direction.
* Take an air filled balloon. Prick it with a needle. Does the air rush out?
Yes
* Fill an open vessel with water. Press the surface of the water with your hands. How do you feel?
Water overflows.
Look at the pictures. Write down what you see.
(Wood, Leaves, Paper)

Match the following.















