Science : Term 1 Unit 4 : Atomic Structure
Evaluation
I. Choose the appropriate answer.
1. The basic unit of matter is ________
a. Element
b. Atom
c. Molecule
d. Electron
Answer: b. Atom
2. The subatomic particle revolve around the nucleus is _______
a. Atom.
b. Neutron
c. Electron.
d. Proton
Answer: c. Electron
3. _______ is positively charged.
a. Protonb.
b. Electron
c. Moleculed.
d. Neutron
Answer: a. Proton
4. The atomic number of an atom is ________
a. Number of neutrons
b. Number of protons
c. Total number of protons and neutrons
d. Number of atoms
Answer: b. Number of protons
5. _______________ Nucleons comprises of
a. Protons and electrons
b. Neutrons and electrons
c. Protons and neutrons
d. Neutrons and Positron
Answer: c. Protons and neutrons
II. Fill in the blanks.
1. The smaller particles found in the atom is called sub-atomic particles.
2. The nucleus has Neutrons and protons.
3. The electrons revolve around the nucleus.
4. If the valency of carbon is 4 and that of hydrogen is 1 , then the molecular formula of methane is CH4.
5. There are two electrons in the outermost orbit of the magnesium atom. Hence, the valency of magnesium is 2.
III. Match the following:
1.Valency – Fe
2. Neutral Particle – Proton
3. Iron – Electrons in the outermost Orbit
4. Hydrogen – Neutron
5. Positively charged Particle – Monovalent
Answer:
1. Valency – Electrons in the outermost Orbit
2. Neutral Particle – Neutron
3. Iron – Fe
4. Hydrogen – Monovalent
5. Positively charged Particle – Proton
IV. True or False. If False, give the correct statement (T/F).
1. The basic unit of an element is molecule. [False]
The basic unit of an element is atom.
2. The electrons are positively charged. [False]
The electrons are negatively charged.
3. An atom is electrically neutral. [True]
4. The nucleus is surrounded by protons. [False]
The nucleus is surrounded by electrons.
V. Complete the analogy.
1. Sun: Nucleus, planets: electrons.
2. Atomic number: number of protons, Mass number: number of protons and neutrons.
3. K: Potassium, C: Carbon.
VI. Assertion and reason.
1. Assertion: An atom is electrically neutral.
Reason: Atoms have equal number of protons and electrons.
2. Assertion: The mass of an atom is the mass of nucleus.
Reason: The nucleus is at the centre.
3. Assertion: The number of protons and neutrons is atomic number.
Reason: The mass number is sum of protons and neutrons.
Answer:
1) A and R True
2) A and R are true but R is not the correct explanation of A
3) A False and R True
VII. Give very short answer.
1. Define an atom.
Matter consists of very small particles which he named atoms. An atom is smallest indivisible particle, it is spherical in shape of a chemical element that retains its chemical properties.
2. Name the sub-atomic particles.
Atoms
|→ Electrons
|→ Protons
|→ Neutrons
3. What is atomic number?
The number of electrons or protons in an atom is called the atomic number of that atom. It is represented by the letter Z.
4. What is the characteristics of proton?
The proton is the positively charged particle and its located in the nucleus. Its positive charge is of the same magnitude as that of the electron’s negative charge.
5. Why neutrons called neutral particles?
The neutron does not have any charge.
VIII. Give short answer.
1. Distinguish Isotopes from Isobar.
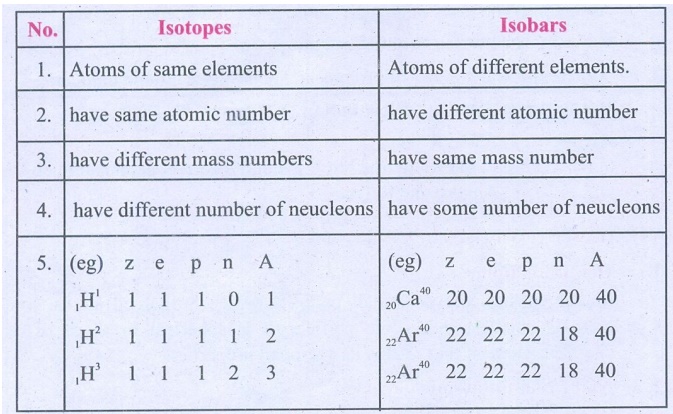
Isotopes
1. Atoms of same elements
2. have same atomic number
3. have different mass numbers
4. have different number of neucleons
5. (eg) z e p n A
1H1 1 1 1 0 1
1H2 1 1 1 1 2
1H3 1 1 1 2 3
Isobars
1. Atoms of different elements.
2. have different atomic number
3. have same mass number
4. have some number of neucleons
5. (eg) z e p n A
20Ca40 20 20 20 20 40
22Ar40 22 22 22 18 40
22Ar40 22 22 22 18 40
2. What are the isotones give one example.
Isotones have same number of neutrons, but different number of protons or electrons.
(e.g) :
1) Boron 5B12 (5e, 5p, 7n)
Carbon 6C13 (6e, 6p, 7n)
Both are having 7 neutrons
(e.g) :
2) Sulphur 16S36 – 16e, 16p, 20n
Chlorine 17Cl37 – 17e, 17p, 20n
Argon 18A38 – 18e, 18p, 20n
Calcium 20Ca40 – 20e, 20p, 20n
All the four are having 20 neutrons.
3. Differentiate mass number from atomic number.
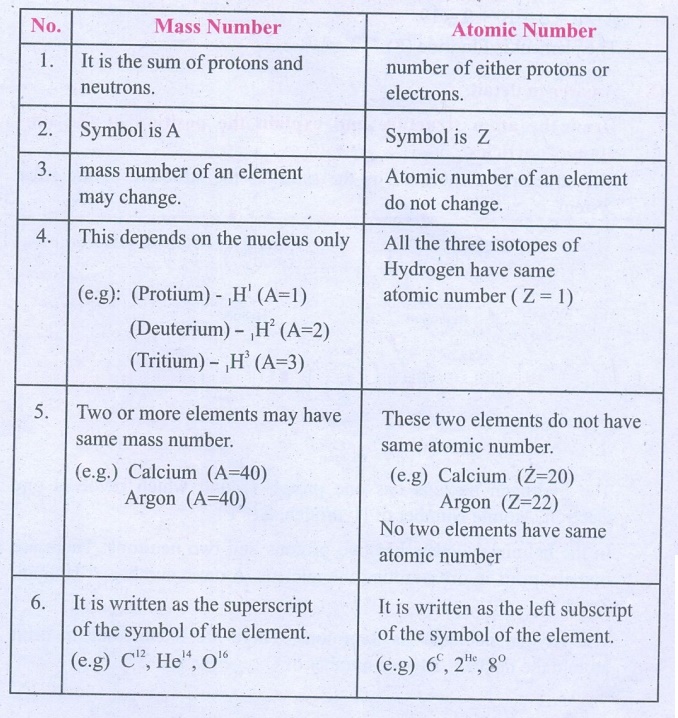
Mass Number
1. It is the sum of protons and neutrons.
2. Symbol is A
3. mass number of an element may change.
4. This depends on the nucleus only
(e.g): (Protium) – 1H1 (A=l)
(Deuterium) – 1H2 (A=2)
(Tritium) – 1H3 (A=3)
5. Two or more elements may have same mass number.
(e.g.) Calcium (A=40), Argon (A=40)
6. It is written as the superscript of the symbol of the element.
(e.g) C12, He14, O16
Atomic Number
1. number of either protons or electrons.
2. Symbol is Z
3. Atomic number of an element do not change.
4. All the three isotopes of Hydrogen have same atomic number (Z = 1)
5. These two elements do not have same atomic number.
(e.g) Calcium (Z=20), Argon (Z=22)
No two elements have same atomic number
6. It is written as the left subscript of the symbol of the element.
(e.g) 6C, 2He, 8°
4. The atomic number of an element is 9, it has 10 neutrons. Find the element from the periodic table. What will be its mass number?
Element is Atomic number: 9
F Automic Mass :19
Fluorine Protons : 9
Neutrons : 10
Electrons : 9
A = p + u = 10 + 9= 19
The element is Fluorine (F)
IX. Answer in detail.
1. Draw the atom structure and explain the position of the sub-atomic particles.
The structure of the atom is the same as the structure of the solar system.
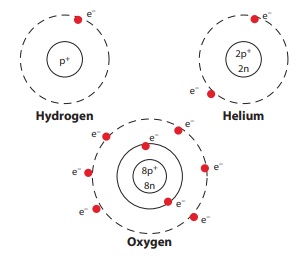
The hydrogen nucleus has one proton around which revolves one electron. Atomic Number of Hydrogen, Z = 1
In the helium neucleus has two protons and two neutrons. There are two electrons in orbit around the nucleus. Atomic number of Helium, Z = 2
The oxygen nucleus has 8 protons. There are 8 electrons in orbit around the nucleus. Atomic number of Oxygen, Z = 8
2. The atomic number and the mass number of an element is 26 and 56 respectively. Calculate the number of electrons protons and neutrons in its atom. Draw the structure.
Atomic number (z) = 26
Mass Number (A) = 26
Number of electrons (e) = 26
Number of protons (p) = 26
Number of neutrons (n) = 30
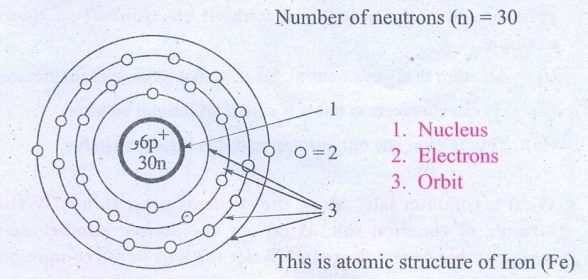
1. Nucleus
2. Electrons
3. Orbit
This is atomic structure of Iron (Fe)
3. What are nucleons. Why are they so called? Write the properties of the nucleons.
Protons and Neutrons are called nucleous. Since these two types of particles are in the nucleus of an atom, they are called nucleons. Proton has positive charge of the same magnitude as that of electrons negative charge. It has one unit of atomic mass. Neutron does not have any charge. It also had one unit of atomic mass.
4. Define valency? What is the valency of the element with atomic number 8. What is the compound by the element with hydrogen.
Valency is defined as the combining capacity of an element. Atoms of different elements combine with each other to form molecules. Valency determines the number of atoms of an element that combines with atom or atoms of another type. Valency of an element depends on the number of electrons in the outer mast orbit of its atom.
This combining property of an atom is called as Valency. It is a measure of how many hydrogen atoms it can combine with For example: oxygen can combine with two hydrogen atoms and create water molecule, the velency of oxygen atom is two. In case of chlorine, it can combine with only one hydrogen to create HC1 m(hydrochloric acid) here the valency of chlorine is one.
X. Questions based on Higher Order Thinking Skills.
1. Anatom of an element has no electron, will that atom have any mass or not? Can atom exist without electron? If so then give example.
(i) An atom is always neutral. So it cannot exist without electrons.
(ii) H+ has no electron but it is called an ion not an atom.
(iii) This H+ ion has only proton and it is highly unstable.
2. Find what is common salt? Name the elements present in it? Write the formula of common salt. What are the atomic number and the mass number of the elements? Write the ions in the compound.
Common salt is sodium chloride with chemical formula NaCl containing two elements Sodium and Chlorine
Sodium Chlorine
Atomic number 11 17
Mass number 23 35
They exist as ions : Sodium ion Na+ and Chloride ion Cl−
XI. Project.
To have an idea of what atoms are, students will construct atoms using pipe cleaners (thin metal wires-electron shells), pom-poms (balls) (different colors for protons and neutrons) and beads (electrons). Students will love and enjoy putting them together and they look great hanging from the ceiling in the classroom.
Student Activities
ACTIVITY 1
Some known objects are shown, also the broken particles of the objects are shown.
1. Name the articles or objects you see here? Also try to write What each of it made of?

1. Hammu (Iron)
2. Bangles (gold)
3. Tap (nickel)
4. Versal (copper)
Now you could imagine how small an atom would be.
ACTIVITY 2
Let us learn the characteristics of the subatomic particles through the following activity. Label the parts in the given diagram and answer the following.
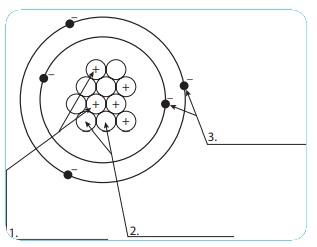
1. The positively charged particleis proton.
2. The negatively charged particleis electron.
3. Electron is neutral.
Try yourself
If the atomic number of carbon is Z=6, what is the number of the electrons revolving in its atom
Try yourself
1. Why are atomic numbers and mass numbers are always whole numbers ?
2. A sulphur atom contains 16 Protons and 16 neutrons . Give its atomic number and atomic mass number.
ACTIVITY 3
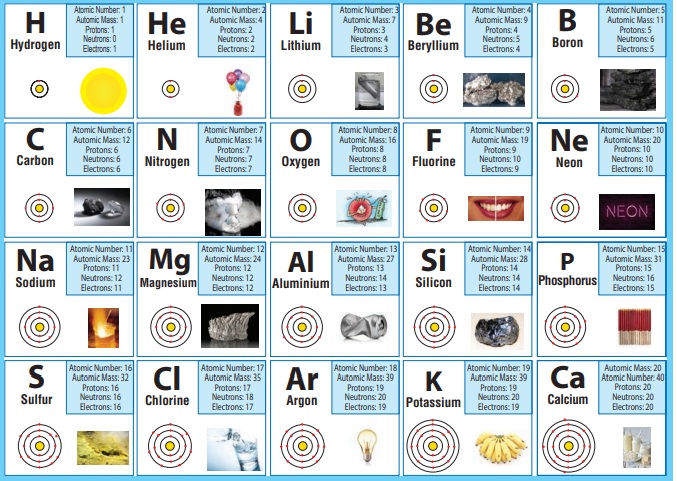
Observe the table given above and answer the following questions.
1. I am used for breathing, without me you cannot live. Do you know me? Write my name and symbol Oxygen, O.
2. It is used in filling the balloons. It is a gas, identity it. What is its mass number? Hydrogen. (A=l).
3. Name the element present in banana. What is my atomic number? Potassium (Z = 19)
4. I am found in crackers. How many protons do i have? Sulfur (p = 16)
5. I am the most valuable element. Find who am I? Can you say my mass number? Carbon (A= 12)














